This chapter is just not an all-inclusive creating on pharmaceutical waters. It contains factors that happen to be fundamental info to generally be regarded as, when appropriate, for that processing, holding, and utilization of water. It is the consumer's duty to assure that pharmaceutical water and its output meet applicable governmental regulations, guidances, as well as compendial requirements to the types of water used in compendial articles.
Surface Houses are regarded with escalating interest given that their options meet the necessities in perspective of far more reputable in vitro tests according to 3D aggregates, an modern method as compared with conventional types [27,28]. Spheroids, which might be three-dimensional aggregates of cells, supply a much more physiologically applicable model for studying cell actions in comparison with classic two-dimensional cultures.
You can also find other types of water for which there aren't any monographs. These are all bulk waters, with names presented for descriptive purposes only.
You can also find other types of water for which there aren't any monographs. These are typically all bulk waters, with names given for descriptive purposes only. Quite a few of those waters are used in particular analytical methods. The involved text may not specify or imply specified high quality attributes or modes of preparing. These nonmonographed waters may well not necessarily adhere strictly for the mentioned or implied modes of preparing or attributes.
USP moved away from these chemical attribute checks to modern day analytical technologies for the majority waters Purified Water and Water for Injection. The intent was to up grade the analytical systems without tightening the quality demands. The two up to date analytical technologies used ended up TOC and conductivity.
“Instrumental” Ways Samples of instrumental approaches include things like microscopic Visible counting strategies (e.g., epifluorescence and immunofluorescence) and very similar automated laser scanning strategies and radiometric, impedometric, and biochemically primarily based methodologies. These approaches all have a number of pros and cons. Positive aspects could possibly be their precision and precision or their speed of check outcome availability in comparison with the classical cultural method. Normally, instrument strategies usually Use a shorter guide time for getting final results, which could facilitate timely process control.
The rationale used by USP to determine its conductivity specification took into account the conductivity contributed by the two least conductive previous characteristics of Chloride and Ammonia, thereby precluding their failure had those damp chemistry tests been executed. In essence, the Stage 3 conductivity specs (see Water Conductivity
The development of RO units that could tolerate sanitizing water temperatures as well as operate proficiently and constantly at elevated temperatures has added considerably for their microbial Handle also to the avoidance of biofouling.
Supplies of design need to be selected to generally be suitable with Regulate measures like sanitizing, cleaning, and passivating. Temperature ranking is actually a vital Consider selecting suitable elements for the reason that surfaces could be needed to handle elevated operating and sanitization temperatures. Ought to chemicals or additives be used to scrub, Handle, or sanitize the program, resources immune to these substances or additives has to be utilized. Materials must be effective at handling turbulent circulation and elevated velocities with out use of your corrosion-resistant movie including the passive chromium oxide area of chrome steel. The complete on metallic products for example stainless-steel, whether it's a refined mill finish, polished to a particular grit, or an electropolished therapy, should really complement program structure and supply satisfactory corrosion and microbial action resistance together with chemical sanitizability.
These methods call for frequent sanitization and microbiological monitoring to guarantee water of correct microbiological good quality with the points of use. The Purified Water monograph also enables bulk packaging for professional use elsewhere. When This can be carried out, the required specifications are These with the packaged water Sterile Purified Water, apart from Sterility and Labeling. There's a possible for microbial contamination as well as other excellent adjustments of the bulk packaged nonsterile water to manifest. For that reason, this way of Purified Water should be geared up and saved in this kind of style that restrictions microbial growth and/or just used inside of a well timed manner right before microbial proliferation renders it unsuitable for its intended use. Also with regards to the content used for packaging, there might be extractable compounds leaching into your water from the packaging. While this article could meet its essential chemical characteristics, these types of extractables may render the water an inappropriate choice for some purposes. It's the consumer's responsibilitiy to guarantee Exercise for use of this packaged article when used in production, scientific, or analytical apps wherever the pure bulk type of the water is indicated.
Creation of pharmaceutical water employs sequential device functions (processing steps) that handle particular water high quality attributes and defend the operation of subsequent procedure actions. A typical evaluation course of action to pick an appropriate water good quality for a particular pharmaceutical goal is proven in the choice tree in Figure 2. This diagram may be used to help click here in defining specifications for unique water employs As well as in the selection of unit functions. The final unit operation used to make Water for Injection is limited to distillation or other procedures equal or superior to distillation within the elimination of chemical impurities in addition to microorganisms and their elements. Distillation has an extended historical past of reputable effectiveness and might be validated being a unit Procedure for the creation of Water for Injection, but other technologies or combos of technologies could be validated as getting equivalently effective. Other technologies, including ultrafiltration next other chemical purification system, may be suitable within the production of Water for Injection if they are often revealed via validation for being as powerful and reputable as distillation. The advent of new products for older systems, such as reverse osmosis and ultrafiltration, that permit intermittent or continual operation at elevated, microbial temperatures, demonstrate promise for a legitimate use in developing Water for Injection.
Here is how you are aware of Official Sites use .gov A .gov Web page belongs to an Formal govt Group in the United States.
This technology get more info could possibly be appropriate as an intermediate or closing purification move. Much like RO, successful functionality is dependent on pretreatment of the water by upstream unit operations.
Macrophages, a type of immune cell, Enjoy a big function in the human body’s response to foreign surfaces. When supplies are introduced into the human body, macrophages are amongst the primary cells to communicate with them. These interactions may result in inflammation and international human body reactions, and also eventually deciding the good results or failure of implanted elements [eleven].
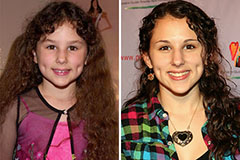 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Kirk Cameron Then & Now!
Kirk Cameron Then & Now! Bug Hall Then & Now!
Bug Hall Then & Now! Michael Jordan Then & Now!
Michael Jordan Then & Now! Peter Billingsley Then & Now!
Peter Billingsley Then & Now!